Introduction of thermodynamic process :
Thermodynamic process :
When the system undergoes a change from one thermodynamic state to final state due change in properties such as temperature, pressure, and volume etc the system is said to have undergone the thermodynamic process. Types of the thermodynamic process described below.
In simple word, a thermodynamic process occurred when the system changes from initial state to the final state.
- Process - Adiabatic
- Process - Isenthalpic
Properties held constant - Enthalpy
- Process - Isentropic
Properties held constant - Entropy, Heat energy, Equilibrium
- Process - Isobaric
Properties held constant - Pressure
- Process - Isochoric
Properties held constant - Volume
- Process - Isothermal
Properties held constant - Temperature
- Process - Isotropic
Properties held constant - Direction
- Process - Polytropic
Properties held constant - PVn = C
- Process - Reversible
Adiabatic process:
An adiabatic process occurs when no heat can flow between a thermodynamic system and its surroundings.
In this process Q = 0.
Example - Vertical flow of air in the atmosphere, Air expands and cools as it rises, and contracts and grows warmer as it descends.
Isenthalpic process :
An isenthalpic process is also called isoenthalpic process. It is a thermodynamic process in which enthalpy is constant.
In this process H = 0.
Example - Throttling process, consider the lifting of a relief valve or safety valve on a pressure vessel.
Isentropic process :
An isentropic process is an idealized thermodynamic process in which both adiabatic and reversible.
It is a process in which entropy remains constant.
In this process ΔS = 0.
Example - Some isentropic thermodynamics device such as pumps, gas compressors, turbines, nozzles, diffusers.
Isothermal process :
An isothermal process is a change of a system, in which the temperature of the system stays constant but heat may flow in or out of the system during an isothermal process.
In this process ΔT = 0.
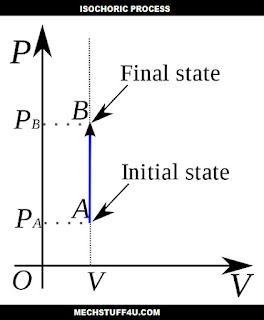
Isochoric process :
An isochoric process as the name suggests iso means same and choric means volume also called constant-volume process or isovolumetric process or isometric process.
It is a thermodynamic process during which the volume of the closed system is kept constant.
In this process ΔV = 0.
Example - Heating of a gas in a closed cylinder.
An isobaric is a thermodynamic process where the pressure of the system stays constant.
Isobaric process :
An isobaric is a thermodynamic process where the pressure of the system stays constant.
In this process ΔP = 0.
Example: Heating of water in an open vessel and the expansion of a gas in a cylinder with a freely moving piston.
Isotropic process :
The isotropic process is one that the permittivity ε and permeability μ of the medium is uniform in all directions of the medium.
Example - Glass and metals are examples of isotropic materials.
Reversible processes :
A reversible process is a process whose direction can be reversed by including infinitesimal changes to some property of the system via its surroundings. In thermodynamics, throughout the entire process, the system is in thermodynamic equilibrium with its surroundings.
Example - Frictionless relative motion, and expansion and compression of spring.
Polytropic Process :
A polytropic is a thermodynamic process that obeys the relation where p is the pressure, V is volume, n is the polytropic index and C is a constant. The equation of this process describes multiple expansion and compression processes which include heat transfer.
PVn = C
From this relationship, we can arrive at relationships for several other types of a thermodynamic process.
- When n = 0 the process is isobaric
- When n = 1 the process is isothermal
- When n = k the process is isentropic
- When n = ∞ the process is isochoric
Example - Expansion of the combustion gasses in the cylinder of a water-cooled reciprocating engine.