In this article, we have to discuss plasma arc machining how it works and its application and main advantages and also disadvantages of this process.
PAM :
When a flowing gas is heated sufficiently high temperature to become partially ionized, it is known as 'plasma'. This virtually a mixture of free electrons, positively charged ions and neutral atoms.
PAM is a material removal process in which the material is removed by ionized gas of high temperature (11,000 to 30,000 C) which applied by a high-velocity jet on the workpiece.
Working principle :
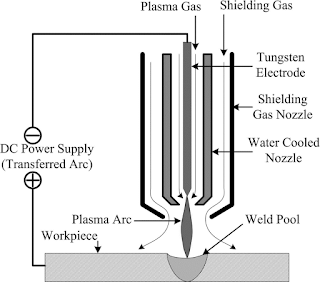
The principle of plasma arc machining is in a plasma torch, known as the gun or plasma-Tron, a volume of gas such as H2, N2, O2, etc. is passed through a small chamber in which a high-frequency spark (arc) is maintained between the tungsten electrode (cathode) and the copper nozzle (anode), both of which are water-cooled. In certain torches, an insert gas-flow surface rounding the main flame is provided to shield the gas from the atmosphere. The high-velocity electrons generated by arc collide with the gas molecules and produce dissociation of diatomic molecules of the gas resulting in ionization of the atoms and causing large amounts of thermal energy to be liberated. The plasma forming gas is forced through a nozzle duct of the torch in such a manner as to stabilize the arc. Much of the heating of the gas takes place in the constricted region of the nozzle duct resulting into relatively high exit gas velocity and very high core temperature up to 30,000 C. the plasma jet steams effectively blows the material and the high-velocity gas stream effectively blows the molten metal away.
Accuracy:
Applications of PAM:
Advantages :
PAM :
When a flowing gas is heated sufficiently high temperature to become partially ionized, it is known as 'plasma'. This virtually a mixture of free electrons, positively charged ions and neutral atoms.
PAM is a material removal process in which the material is removed by ionized gas of high temperature (11,000 to 30,000 C) which applied by a high-velocity jet on the workpiece.
Working principle :
The principle of plasma arc machining is in a plasma torch, known as the gun or plasma-Tron, a volume of gas such as H2, N2, O2, etc. is passed through a small chamber in which a high-frequency spark (arc) is maintained between the tungsten electrode (cathode) and the copper nozzle (anode), both of which are water-cooled. In certain torches, an insert gas-flow surface rounding the main flame is provided to shield the gas from the atmosphere. The high-velocity electrons generated by arc collide with the gas molecules and produce dissociation of diatomic molecules of the gas resulting in ionization of the atoms and causing large amounts of thermal energy to be liberated. The plasma forming gas is forced through a nozzle duct of the torch in such a manner as to stabilize the arc. Much of the heating of the gas takes place in the constricted region of the nozzle duct resulting into relatively high exit gas velocity and very high core temperature up to 30,000 C. the plasma jet steams effectively blows the material and the high-velocity gas stream effectively blows the molten metal away.
Accuracy:
- This is a roughing operation to an accuracy of about 15 mm with a corresponding surface finish.
- Accuracy on the width of slots and diameter of holes is ordinary from +0.8 mm to -0.9 mm on 6 to 30 mm thick plates, and +3 mm to -3 mm on 100 to 150 mm thick plates.
- This is chiefly used to cut stainless steel and aluminium alloys.
- Profile cutting of metals, particularly of these metals and alloys, has been the most prominent commercial application of PAM.
- PAM has been used successfully in turning and milling of materials which are hard and difficult to machine.
Advantages :
- The main advantages of this process are that it is almost equally effective on any metal, regardless of its hardness of refractory nature.
- There being no contact between the tool and work piece, only a simply supported work piece structure is enough.
Disadvantages :
- The main disadvantages of this process are the metallurgical change of the surface.
- Safety precautions are necessary for the operator and those in near by areas.
- This adds additional cost.